How does a conductivity detector work and why is it essential for accurate measurements?
As significant instruments in analytical chemistry, environmental monitoring, and industrial processes, these sophisticated detectors measure the electrical conductivity of solutions, thereby providing data for quality control, research, and regulatory compliance. With an understanding of the actual workings, one would be able to take measurements with more precise data and make a considerable number of decisions across various scientific disciplines.
Understanding Conductivity and Its Importance
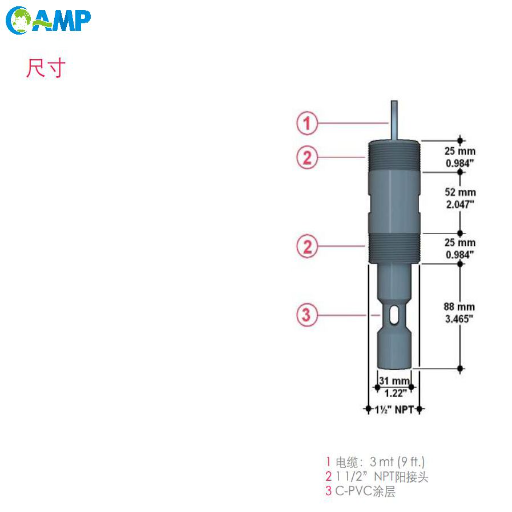
What is Electrical Conductivity?
Electrical conductivity is the property of a material by which it allows the passage of electric current through it. In solution, conductivity depends directly on the concentration of dissolved ions, the positively and negatively charged particles that act as charge carriers. In mixtures containing dissolved salts, acids, bases, and other ionic species, water dissociates into positive and negative ions, providing a path for electrical current.
When the concentration of ions increases, the solution's conductivity increases. In contrast, pure water has very low conductivity because it contains very few ions. This fundamental relationship with conductivity provides a method for crude analysis of solution composition and purity.
Conductivity measurements are expressed in units of siemens per meter (S/m) or, for dilute solutions, microsiemens per centimeter (μS/cm). The reciprocal of conductivity, resistivity, is measured in ohm-meters (Ω⋅m).
Some Applications of Conductivity Measurements
Conductivity measurements are applied in numerous fields. Environmentalists use conductivity measurements to assess water quality, pollution levels, and ecosystem viability. Conductivity measurement in industry is used to provide process control, ensuring the desired product specifications are met at all times.
Conductivity, as an indicator of total dissolved solids, is used in water treatment plants for adjusting treatment processes. In the pharmaceutical and food industries, conductivity measurements are used to assess product purity and quality during production.
Factors Affecting Conductivity
Temperature greatly influences conductivity measurements; ionic mobility increases with rising temperature, leading to higher conductivity. Most conductivity meters employ automatic temperature compensation to correct such variations and give standard readings.
Other parameters that affect conductivity include the type and concentration of dissolved substances. Strong electrolytes such as sodium chloride contribute more to conductivity than weak electrolytes at the same concentration. Moreover, physical parameters of the liquid like viscosity and density would also affect the accuracy of the measurements.
The Basics of Conductivity Sensors

What is a Conductivity Sensor?
A conductivity sensor, also known as a conductivity probe or electrode, is basically the interface between the measurement electronics and the solution under analysis. Such sensors have electrodes that apply a known voltage across the solution and measure the resulting current, with conductivity computed using Ohm's law.
Modern conductivity sensors are highly engineered to reduce measurement errors and increase lifespan. The cell constant of the sensor, a geometrical factor that depends on electrode spacing and surface area, is considered an important factor in converting measured electrical parameters into a conductivity value.
Different kinds of Conductivity Probes
Many configurations of conductivity sensors exist, each optimized for specific measurement ranges and applications. Contacting conductivity sensors make physical contact with a solution via electrodes, while non-contacting conductivity sensors measure it via electromagnetic induction without any contact.
They are further classified by contact type based on their electrode configuration. The two-electrode type is simple in design and is suitable for a variety of applications. However, the four-electrode type is exceptionally accurate because it can separate the current-carrying function from the voltage-sensing function.
Glass-bodied types exhibit high chemical resistance and are thus suitable for harsh environments, whereas those with plastic or metal housings are designed for industrial use. Meanwhile, other designs include insertion probes for process applications, flow-through cells used for continuous monitoring, and portable sensors earmarked for field measurements.
How Conductivity Sensors Function
The operation of conductivity sensors is based on applying an AC voltage between electrodes immersed in the test solution. Alternating current is used to prevent electrode polarization, a phenomenon that could lead to measurement errors if direct current were used.
When voltage is applied, current passes through the solution in a proportion determined by its conductivity. The measuring electronics of the sensor monitor this current so the conductivity can be calculated with respect to the voltage and current readings and the cell constant of the sensor.
Nowadays, some of the modern sensors use several frequencies to compensate for characteristics of the solution so that these conditions are not affecting the accuracy of the measurement. In this way, a real ionic conductivity can be separated from the capacitive effects that might interfere with an analysis.
Working Principles of Conductivity Detectors
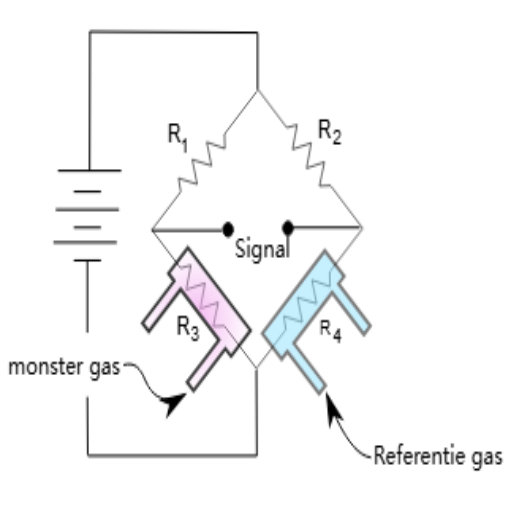
Two- and Four-Electrode Sensors
Two-electrode sensors are the most straightforward form of conductivity sensors. The instrument applies voltage between two electrodes and measures the current. In many cases, the system is fine. However, it is worth noting that two-electrode sensors are somewhat affected by electrode polarization and contact resistance, especially in high-conductivity solutions.
Four-electrode sensors overcome these problems by using separate electrode pairs for current generation and voltage measurement. The two outer electrodes carry the measurement current, while the two inner electrodes sense the voltage drop across the solution. This measurement eliminates electrode contact resistance, thereby achieving greater accuracy over a wide range of conductivities.
The four-electrode arrangement is very important, especially when dealing with solutions of conductivity higher than 1000 μS/cm, whenever two-electrode measurements are severely affected by electrode effects. Many modern conductivity meters automatically switch between two- and four-electrode modes based on the measured conductivity.
Calibration of Conductivity Meter
Conductivity meters require calibration with standard solutions of known conductivity for accurate measurements. Usually, standard solutions are prepared from known concentrations of potassium chloride (KCl) for a stable, reproducible conductivity reference.
Some common calibration standards are: 0.01 M KCl (1413 μS/cm), 0.1 M KCl (12,880 μS/cm), and 1.0 M KCl (111,800 μS/cm) at 25°C. It depends on the approximate value expected to be measured in the samples under analysis to determine which calibration standard should be used.
Calibration is usually done by simply plunging the sensor into the standard solution, waiting for temperature equilibration, and adjusting the meter reading until it matches the standard value. Multi-point calibration with several standards ensures greater accuracy across measurement ranges.
Conductivity Measurement Techniques
The modern conductivity detector employs even more sophisticated techniques to ensure measurement accuracy and reliability. The pulse techniques maintain the precision of the measurement while the short voltage pulses tend to minimize electrolyte polarization. The detector determines the current response for a certain time interval within every pulse cycle.
Frequency measurements use multiple AC frequencies to provide a more complete characterization of the solution's properties. Different frequencies reveal different properties of ion mobility, electrode effects, and solution capacitance so that the detector can compensate for interferences in measurement.
DSP filters and computes average from many samples to increase measurement stability. Being so intelligent, the new instruments adjust measurement parameters based on solution characteristics, thereby achieving the best accuracy for each application.
Factors Influencing Conductivity Measurements

Effect of Ion Concentration
The ion concentration and conductivity relationships different from solution to solution against the predictable pattern. At a lower concentration, the increased number of ions leads to an increase in conductivity linearly with concentration. However, the high concentration allows inter-ionic interactions, leading to deviations from linearity.
Different ions contribute differently to the solution's conductivity depending on their charge and mobility. Those ions that are highly mobile in the solution, such as hydrogen and hydroxide ions, tend to contribute much more to the conductivity than those ions that are bigger and less mobile at the same concentration.
Given the established relationship between conductivity and ion concentration, one can estimate ion concentrations from conductivity measurements; however, direct quantitative analysis requires calibration against a known composition.
Effect of Temperature on Conductivity
Temperature affects conductivity measurements via several mechanisms. As the temperature rises, ions move faster, and thus conductivity increases at about 2-3% per each degree Celsius in most solutions. Temperature also affects the degree of dissociation of weak electrolytes, thereby altering conductivity.
Temperature is measured in an ATC system simultaneously with conductivity, and correction factors are applied to compensate the conductivity reading to a standard temperature, most commonly 25°C. In this way, measurements remain consistently comparable regardless of changes in the sample's temperature.
For specialized conditions, these detectors allow one to manually adjust the temperature compensation coefficient to the exact solution properties to obtain highest accuracy.
Understanding Salinity and Resistivity
Salinity measurements used in oceanographic and environmental applications are derived from conductivity measurements using various accepted conversion algorithms. The relationship between conductivity and salinity accounts for the ionic composition of seawater and related natural waters.
Resistivity, the inverse of conductivity, provides an alternate means to express the characteristics of a solution. Pure water applications employ resistivity measurements, since even minute changes in purity produce large, easily measured deviations in resistivity.
The choice between reporting conductivity and resistivity depends on the application and the typical range of encountered values. Conductivity is reported for solutions that have a significant ionic content, while resistivity is near to being a standard report for high-pure applications.
Applications of Conductivity Detectors in Chemical Analysis
Environmental monitoring
Though one of the prime cases of application of conductivity detectors, environmental monitoring is the general term for an array of activities conducted to assess water quality to determine ecosystem health and pollutant sources. Natural waters show a particular range of conductivity, and distinguishing from that range is often a matter of contamination or other form of environmental insult.
Just for example, conductivity measurements are used in groundwater monitoring to trace saltwater intrusion into coastal aquifers; assess landfill leachate impact on water quality, and evaluate the effectiveness of remediation measures. In contrast, real-time conductivity monitoring provides the early warning of the contamination event to initiate rapid response for protection of water resources.
Also, the surface water monitoring utilizes conductivity measurements to evaluate influences of agricultural runoff, compliance with industrial discharge, and seasonal fluctuations in water quality. Automated stations equipped with conductivity monitors generate continuous data for environmental management decisions.
Industrial Applications
With great applications in industries on conductivity measurement, applications can be seen in quality control and process optimization. Chemical industries measure conductivity to verify reaction progress, product specifications, and separation operations.
Preventing corrosion and scaling in critical equipment by monitoring boiler water conductivity is a power generation application. Protection of turbines and other high-value components from contamination damage is by monitoring steam purity with conductivity detectors.
Semiconductor manufacturing requires the use of ultra-pure water with very low conductivity. Monitoring conductivity throughout the process ensures the requirement of water being met so that the defect cannot creep into the sensitive electronic device.
Water quality control in water treatment
Water treatment plants have conductivity monitors at various locations throughout the treatment process. Raw water is monitored to provide baseline information for treatment planning, while intermediate values help assess treatment effectiveness at any stage.
Reverse osmosis is an example where conductivity monitoring is employed—interrupting assessments of how well the membranes perform and developing cleaning schedules for them. Any sudden increase in permeate conductivity is a warning of potential membrane failure and should be addressed preemptively before the system becomes seriously compromised.
Conductivity measurements are made at various points in the distribution network to detect contaminations, prove disinfection, and enforce standards. Automated monitoring systems shall alert personnel in real time when conductivity exceeds preset limits.
Recently Posted
-
Understanding the Functions of Suction Valve and Discharge Valve in Pump Systems
December 18, 2025Industrial pump systems rely on precise mechanical coordination to transport fluids effectively. At the heart of this process are Read More
Read More -
Exploring Advanced Technologies in Air Compressor Discharge Valve Manufacturing
December 17, 2025The efficiency of any pneumatic system relies heavily on the performance of its smallest components. Among these, the air compress Read More
Read More -
Exploring Different Discharge Valve Types for Efficient Fluid Flow Management
December 16, 2025Industrial systems rely heavily on precision components to maintain safety, efficiency, and operational integrity. Among these com Read More
Read More -
How the Discharge Valve AC Affects Performance and Longevity of Cooling Systems
December 15, 2025The efficiency of any air conditioning system relies heavily on the precise coordination of its internal components. While the com Read More
Read More






















